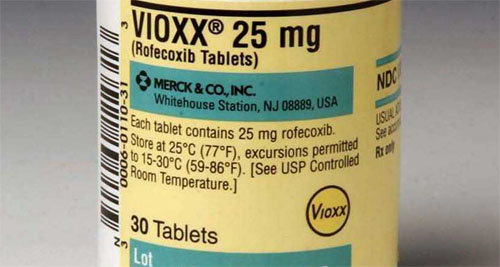
The startup Tremeau Pharmaceuticals is working to bring back the once popular, yet controversial arthritis pill, Vioxx. However, they plan on marketing it to treat “severe joint pain caused by the bleeding disorder hemophilia.”1 But, if it’s approved doctors could “legally prescribe it to anyone.”2 (Back in 2004, Merck & Co. voluntarily pulled the drug because of evidence that it doubled the chances of having a heart attack or stroke.)
When Merck ended Vioxx production they were facing thousands of lawsuits from people who claimed the drug had caused their heart attacks or strokes. But this was no surprise; Merck knew about the risks because their own research showed the drug doubled those risks. However, patients believed the company had downplayed or concealed that fact and Merck would go on to pay a $4.85 billion settlement.
Because most pain relievers increase the risk of internal bleeding, many hemophilia patients rely on opioid painkillers. However, as opioids are incredibly dangerous and habit-forming finding an alternative would be beneficial for the hemophilia community. Enter Vioxx. (Hemophilia is an inherited disorder where the body is missing specific proteins in the blood needed for clotting. This means that even the slightest injury can trigger uncontrolled internal bleeding. In the U.S. there are a little more than 20,000 people with the disorder.)
RELATED STORY:
Tremeau’s chief executive, Brad Sippy, who was once a pharmaceutical marketing executive at Merck during the Vioxx years, believes that Vioxx can fix this “huge unmet medical need.” And so, with the knowledge that the final patent protecting Vioxx’s monopoly was expiring this fall he “put together a plan and co-founded Tremeau last year to develop nonopioid pain treatments for rare diseases.”3
If Sippy gets approval to sell rofecoxib (Vioxx’s chemical name) doctors could once again prescribe it to anyone with chronic pain. While Tremeau wouldn’t be legally able to promote unapproved uses it’s inevitable that some patients would want it (Vioxx was so effective that people “hoarded it” after Merck removed it from the market). However, the drug would carry a strong warning about the heart attack and stroke risks. It would be left up to doctors to be wise about who they prescribed it to and how much they educated them about the serious risks.
RELATED STORY:
Tremeau must still raise at least $25 million in order to test the drug in hemophilia patients. Then, the results must speak for themselves in order to receive FDA approval.
RELATED STORY: