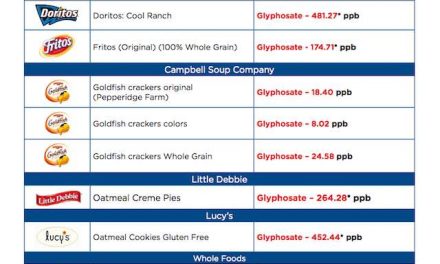
The FDA, U.S. Food & Drug Association, has requested that Endo Pharmaceuticals remove their reformulated Opana ER (oxymorphone hydrochloride) opioid pain medication from the market. This is the first time the FDA has ever asked for the removal of a previously approved medication.
RELATED ARTICLES:
- Unless You’d Consider Heroin, Don’t Take This Prescription Drug
- Opioid Abuse Plummets in States with Legal Marijuana
The FDA found that the risks of Opana ER currently outweigh the benefits because it is an easily abused drug.
FDA Commissioner Scott Gottlieb, M.D. states:
“We are facing an opioid epidemic – a public health crisis, and we must take all necessary steps to reduce the scope of opioid misuse and abuse. We will continue to take regulatory steps when we see situations where an opioid product’s risks outweigh its benefits, not only for its intended patient population but also in regard to its potential for misuse and abuse.”
Opana ER was first approved for use in 2006 for management of moderate to severe pain. In 2012, Endo Pharmaceuticals changed the formulation to make chemical and physical alterations more difficult. This was in an effort to make Opana ER more difficult to abuse. However, the FDA found their steps were not good enough.
Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research states:
“The abuse and manipulation of reformulated Opana ER by injection has resulted in a serious disease outbreak. When we determined that the product had dangerous unintended consequences, we made a decision to request its withdrawal from the market. This action will protect the public from further potential for misuse and abuse of this product.”
Diseases linked to Opana ER abuse include HIV, hepatitis C, and thrombotic microangiopathy, a serious blood disorder.
RELATED ARTICLE:
This two-year-old news report shows how serious this problem is and how long it has been going on.
*Article originally appeared at David Wolfe.